-40%
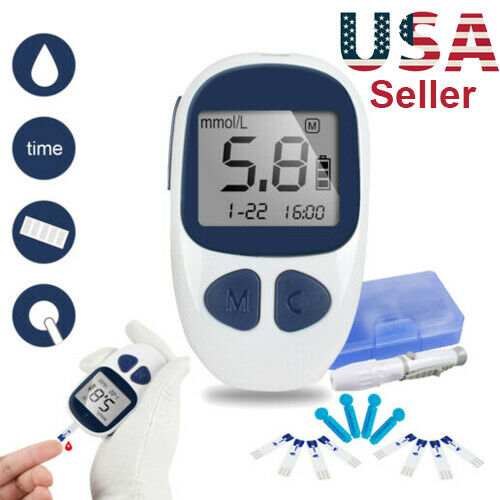
Digital Glucometer Blood Glucose Monitor Diabetes Test Kit With 50Strips Lancets
$ 10.81
- Description
- Size Guide
Description
Description:Features:
Small blood sample: Only 1 micro-liter is required
Fast 6 second test time
Small and erogonomic: easy to carry.
500 test results memory storage
Auto shutdown: automatic shutdown after 2 minutes.
Specification:
Glucose test range: 1.1-33.3 mmol/L
Minimum sample volume: 1μL
Test Time: About 6 seconds
Battery Type:CR 2032 3.0V Coin
Memory storage: 500 test results
Operating temperature: 10-40ºC(50-104ºF)
Operatiog humidity: 20-80%RH
Weight:240g
Package Include:
1×Blood glucose meter
1×Lancing device
50× Test strips
50×
Lancets
FDA declaration
:
The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item.
(
Seller Name:Dorothea;Country: USA;State:
New Jersey
;City:
Jersey City
)
.
This item has been cleaned and treated according to the manufacturer's instructions.
The Fingertip Pulse Oximeter is certified with the US FDA 510K No. K070371, the CE & TUV of Eureope and it is on the Australian Register of Therapeutic Goods (ARTG) with the code 136606.
The Powered Surgical Instrument / Speed 808 System is certified with the US FDA 510(k) Number:K132989
The Powered Surgical Instrument / Hair Remove Device is certified with the US FDA 510(k) Number:K180353
The Powered Surgical Instrument / Hair Remove System is certified with the US FDA 510(k) Number:K141973
massager, vacuum, light induced heating / Slimming Treatment Device is certified with the US FDA 510(k) Number:K161892